Introduction
Have you ever wondered why your rhododendrons thrive effortlessly while your tomatoes struggle despite all the care you give them? In the lush, rainy climate of the Pacific Northwest west of the Cascades, the secret often lies beneath the surface—in your garden soil’s pH levels. As a landscaping enthusiast here at Classic Landscaping + Nursery, I’ve seen countless gardens transformed simply by understanding and tweaking this one factor. Let’s dive into what soil pH really means and how it impacts your plants.
What Exactly Is Soil pH?
Soil pH measures how acidic or alkaline your garden soil is, on a scale from 0 to 14. A pH of 7 is neutral—think pure water. Below 7 means acidic soil, and above 7 indicates alkaline conditions. In our region, with all that rainfall leaching away basic minerals, most soils naturally hover around 5 to 6, making them slightly acidic. That’s perfect for native beauties like Pacific rhododendrons or red flowering currants, but it can spell trouble for other plants.
Imagine pH as the gatekeeper for nutrients. It determines which doors open for your plants to absorb essentials like nitrogen, phosphorus, and potassium. Get it wrong, and even the richest soil won’t deliver what your garden needs.
Why Soil pH Matters in the Pacific Northwest
Here in the PNW, our heavy rains and coniferous forests contribute to naturally acidic soils. This acidity can lock up nutrients, leading to yellowing leaves (chlorosis) or stunted growth. For instance, if your soil dips below 5.5, iron and manganese become more available, but phosphorus might get tied up, starving your veggies.
On the flip side, our acidic environment suits acid-loving plants perfectly. But if you’re growing a diverse garden—say, mixing natives with imported perennials—you might notice imbalances. Low pH can also boost harmful fungi while slowing beneficial bacteria, affecting overall soil health.
Think about it: Have you planted blueberries that exploded with fruit, only to have your roses nearby look lackluster? That’s pH at play. According to Oregon State University Extension, pH levels above 7 are rare west of the Cascades, so we often focus on raising pH for broader plant compatibility.
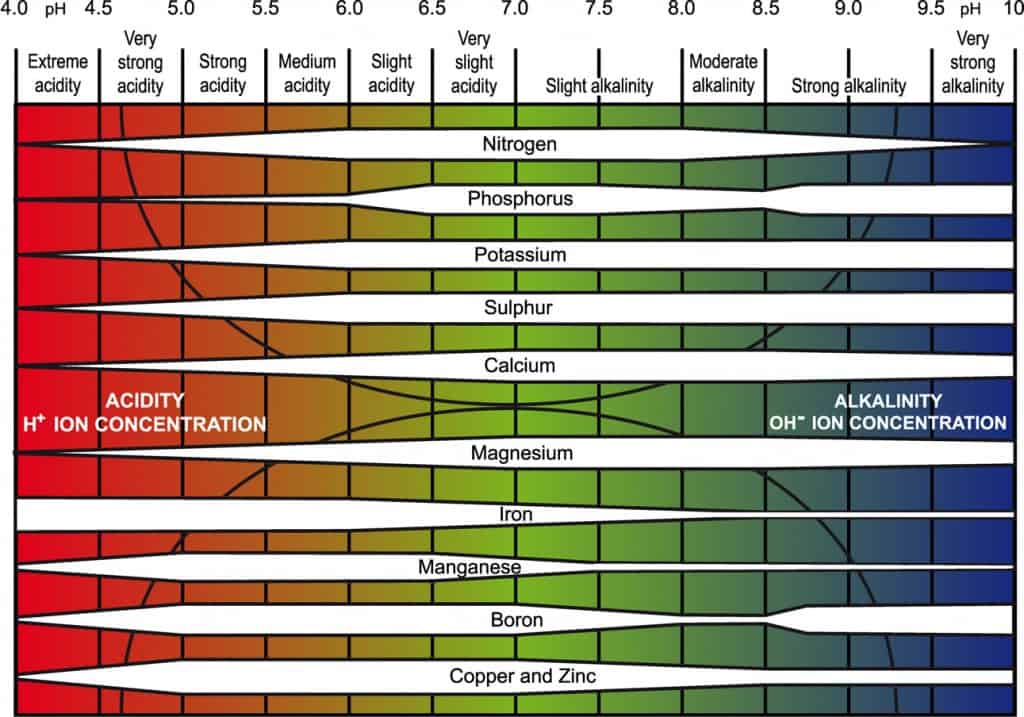
The Impact on Nutrient Availability
Soil pH directly influences how plants access nutrients. In acidic soils common to our area, elements like aluminum can become toxic, stunting roots and interfering with uptake. Meanwhile, key macronutrients thrive in slightly acidic to neutral ranges (6-7).
For example:
- Nitrogen: Best available around 6-8, but our low pH can hinder bacterial fixation.
- Phosphorus: Peaks at 6-7; too acidic, and it binds with iron or aluminum.
- Potassium: Widely available, but extremes reduce it.
A handy chart illustrates this beautifully—nutrients are most accessible in that sweet spot of 6 to 7.5 for most garden plants.
In the PNW, where soils often test at 5-6, you might need to amend for veggies or lawns to hit that optimal range.
Traditional Ways to Test Your Soil pH
Testing is the first step—no guesswork needed. Traditional methods keep it simple and effective.
Start with a home test kit from your local nursery. Collect samples from 6-8 inches deep in vegetable beds or 6-10 inches for shrubs. Mix from several spots for accuracy.
For a DIY approach, try the pantry method:
- Take two tablespoons of soil in separate bowls.
- Add ½ cup vinegar to one—if it fizzes, your soil is alkaline (pH >7).
- Add ½ cup baking soda mixed with water to the other—if it fizzes, it’s acidic (pH <7).
This gives a rough idea. For precision, send samples to a lab like the OSU Extension Soil Testing Lab for detailed results.
We recommend testing annually, especially after heavy rains that can acidify soil further.
Adjusting Soil pH Traditionally
Once tested, adjustment is straightforward with time-tested materials.
To raise pH (common in our acidic PNW soils), apply agricultural lime (calcium carbonate). It’s slow-acting but effective—spread 5-10 pounds per 100 square feet for a 0.5 pH increase, then till in and water well. Dolomitic lime adds magnesium too, great for our nutrient-leached soils.
To lower pH for acid-lovers, use elemental sulfur. It reacts with soil bacteria to produce acid—apply 1-2 pounds per 100 square feet to drop pH by 1 unit. Avoid overdoing it; changes take months.
Incorporate these during fall for spring readiness. For an eco-friendly twist, add organic matter like compost, which buffers pH naturally over time. Check out our sustainability practices for more green tips.
Always retest after amendments. If pests arise from imbalances, our pest control guide has you covered.
Matching Plants to Your Soil pH
Why fight nature? In the PNW, embrace acid-tolerant plants from our plants selection. Rhododendrons (pH 4.5-6), azaleas, and ferns love our native acidity.
For veggies aiming for 6-7, raise beds with lime. Lawns prefer 6.5-7; if yours yellows, pH might be the culprit—consult our landscape care services.
Story time: A client in Seattle had wilting hydrangeas turning pink instead of blue. A quick pH test revealed 6.8—too high. We acidified with sulfur, and now they’re vibrant blue beauties.
Common Mistakes and Pro Tips
Avoid liming without testing—you could overshoot. Don’t mix amendments with fertilizers; apply separately. In our wet climate, raised beds help manage pH by improving drainage.
For hardscape integration, like patios near gardens, ensure soil stability—see our hardscape options.
Ready to Optimize Your Garden?
Understanding pH levels in garden soil is key to a flourishing PNW landscape. Don’t let imbalances hold you back—test today and adjust wisely.
Ready for expert help? Contact Classic Landscaping + Nursery for a consultation. Our design-build services can tailor your space perfectly. Let’s make your garden thrive—reach out now!
:max_bytes(150000):strip_icc()/what-to-know-about-soil-ph-5204392-v1-2813a325b217492fa2fe515e08c38ec8.png)